Dr. Al Benson and I run the Leeds Systems Physiology Lab,
part of the Cardiovascular
Sciences Research group
in the Faculty of Biological Sciences,
University of Leeds.
Our research focuses on dissecting physiological mechanisms in both health and disease, primarily related to cardiac function and exercise physiology.
These pages describe the basics of the underlying biomedical concepts to place the context for our research.
Cardiovascular disease is a leading cause of morbidity and mortality in the developed world, yet current treatment strategies are sub-optimal.
Cardiac dysfunction can be electrical (arrhythmia – where the regular rhythm of the heart is interrupted) or mechanical (where the development of
mechanical contractile force is inhibited); the two are also closely linked to each-other, as the electrical excitation is the local trigger for
mechanical contraction. Due to the substantial socio-economic costs of cardiovascular disease, there is a pressing need to develop greater
understanding of the mechanisms underlying abnormal rhythm and failed contraction in the heart in order to devise more effective diagnostic,
prevention and treatment approaches.
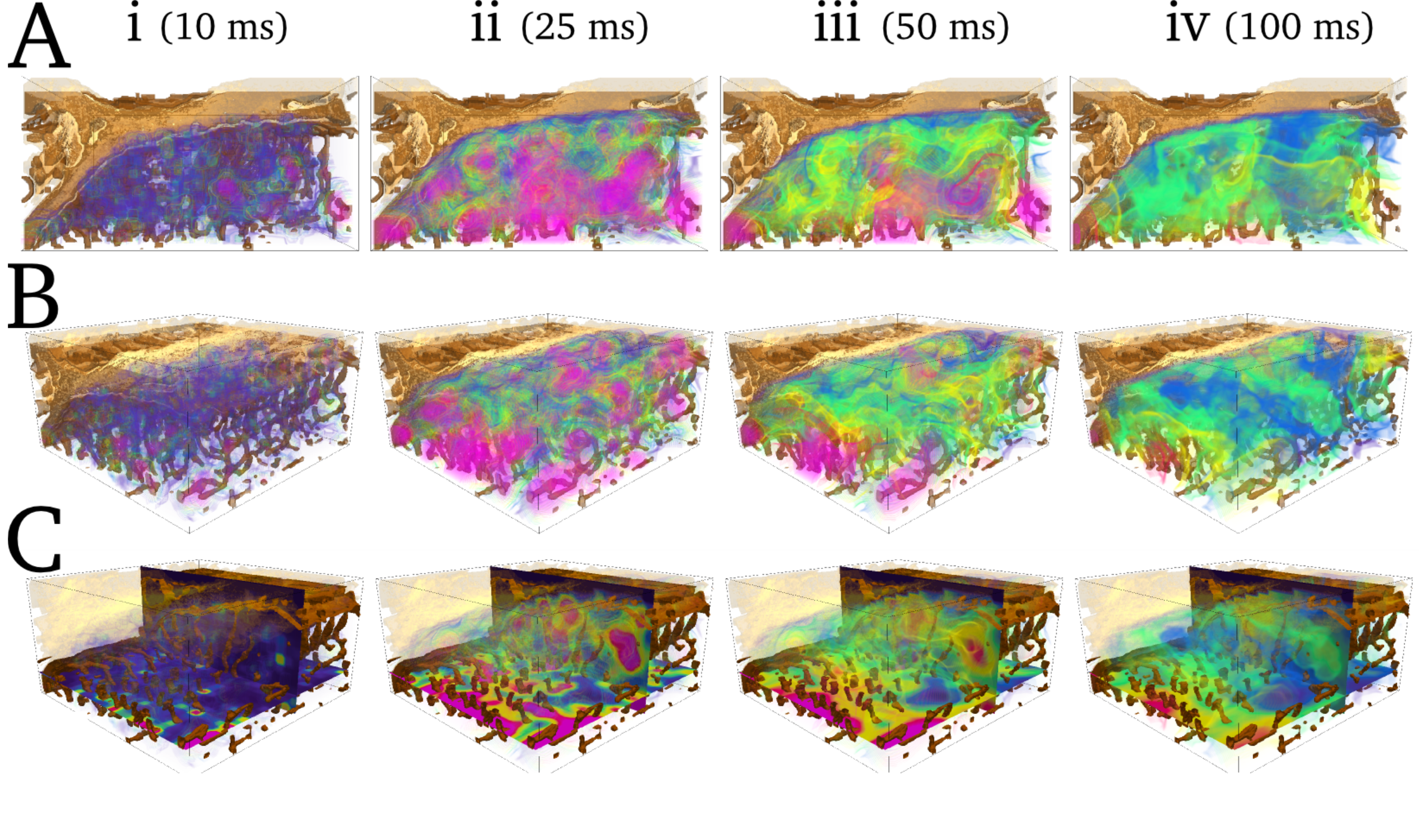
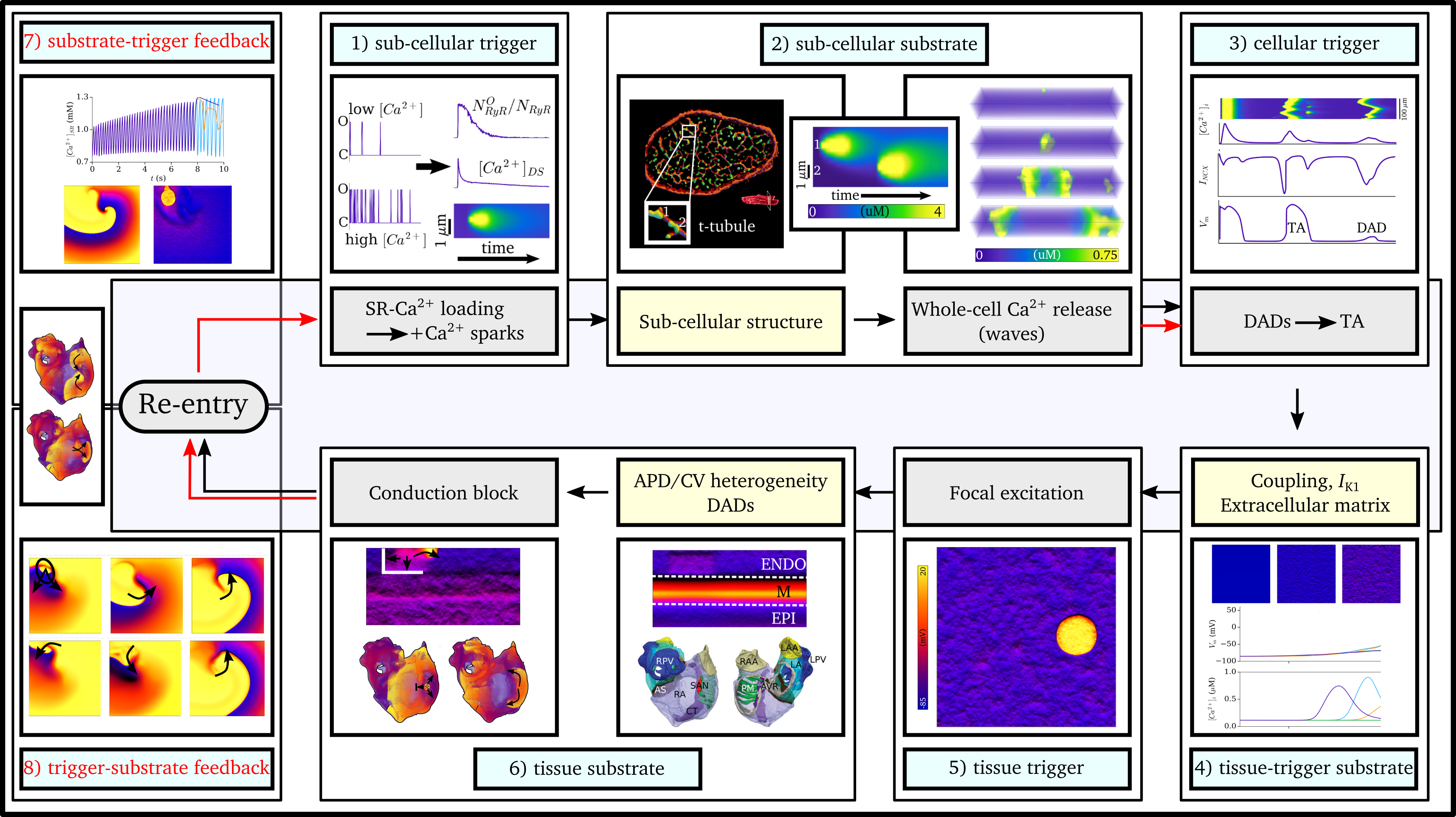
The details of the mechanisms of the transition to- and perpetuation of- arrhythmia are complex and depend on function from the smallest
(single proteins) right up to the largest (whole-heart) scales, making them challenging to understand and manage with pure experimental approaches.
The mechanisms of the coupling between the electrical and mechanical function of the heart are also subject to similar multi-scale considerations,
in particular depending on complex structures from the sub-cellular to the whole-tissue levels.
Computational modelling - simulation of the electrical and mechanical activity of the heart - has become an increasingly powerful tool in the wider effort
to understand, diagnose and treat cardiac disorders. In particular, computational modelling allows true multi-scale investigation, linking behaviour at the
sub-cellular scale to organ scale phenomena. My research interests lie at the interface of physics and biomedical science, in the application of mathematical
and physics techniques currently used for theoretical investigation to develop advanced multi-scale computational frameworks for simulation of cardiac activity.
My research interests are in the development and application of novel multi-scale joint experimental-simulation frameworks to understand the
mechanisms of cardiac electrophysiology, intracellular calcium handling, and excitation-contraction coupling. The aim is to improve understanding of cardiac dysfunction
and ultimately lead to better diagnosis and treatment strategies. Primarily, my focus is on the multi-scale interactions of components
from the sub-micron to the whole-heart scale, and the role of these interactions in the pathologies associated with heart failure, Atrial Fibrillation,
and ageing, regarding arrhythmia and excitation-contraction coupling.
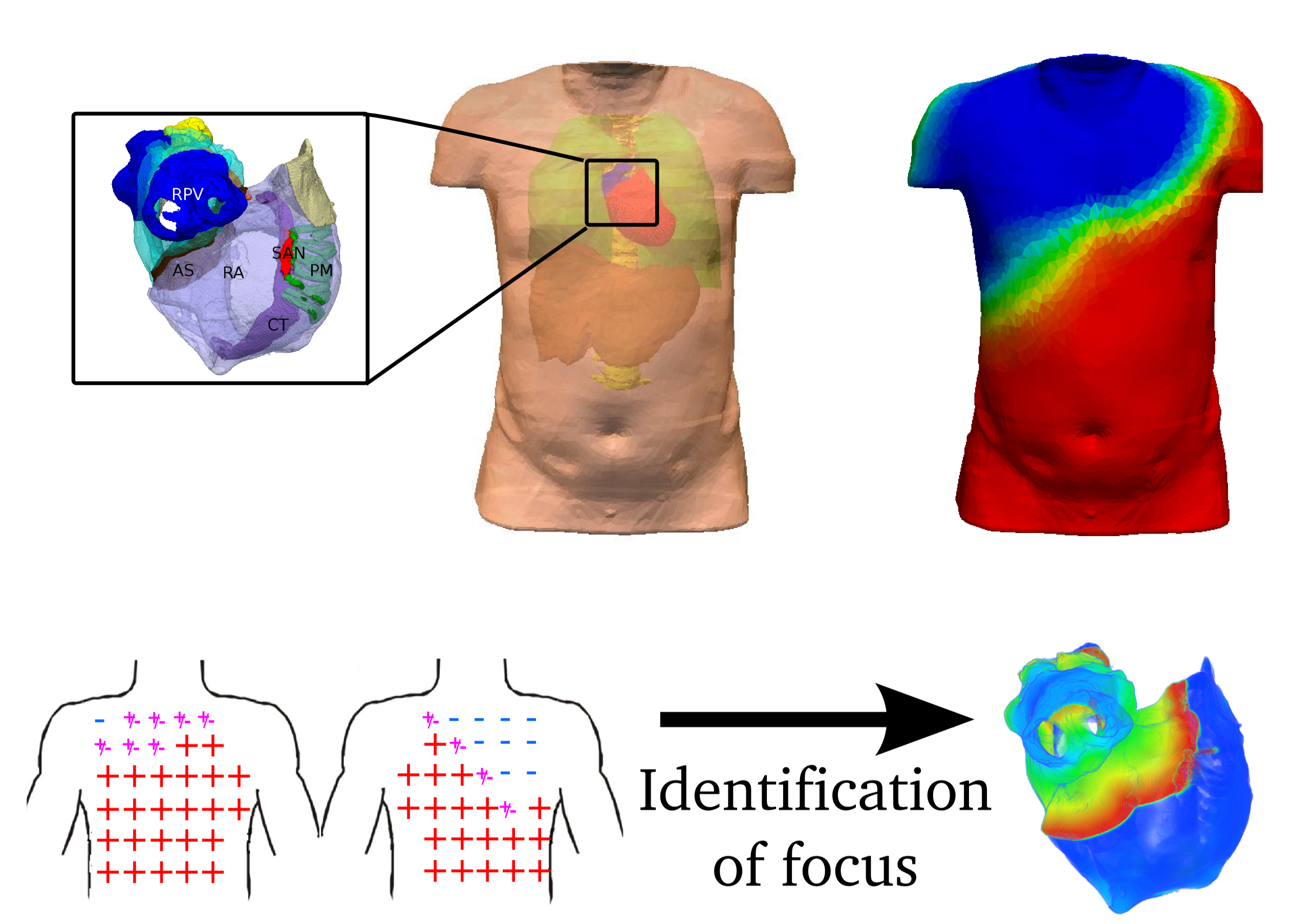
Finally, in order to assist in the development of improved diagnostic strategies, I also have an interest in non-invasive cardiac mapping.
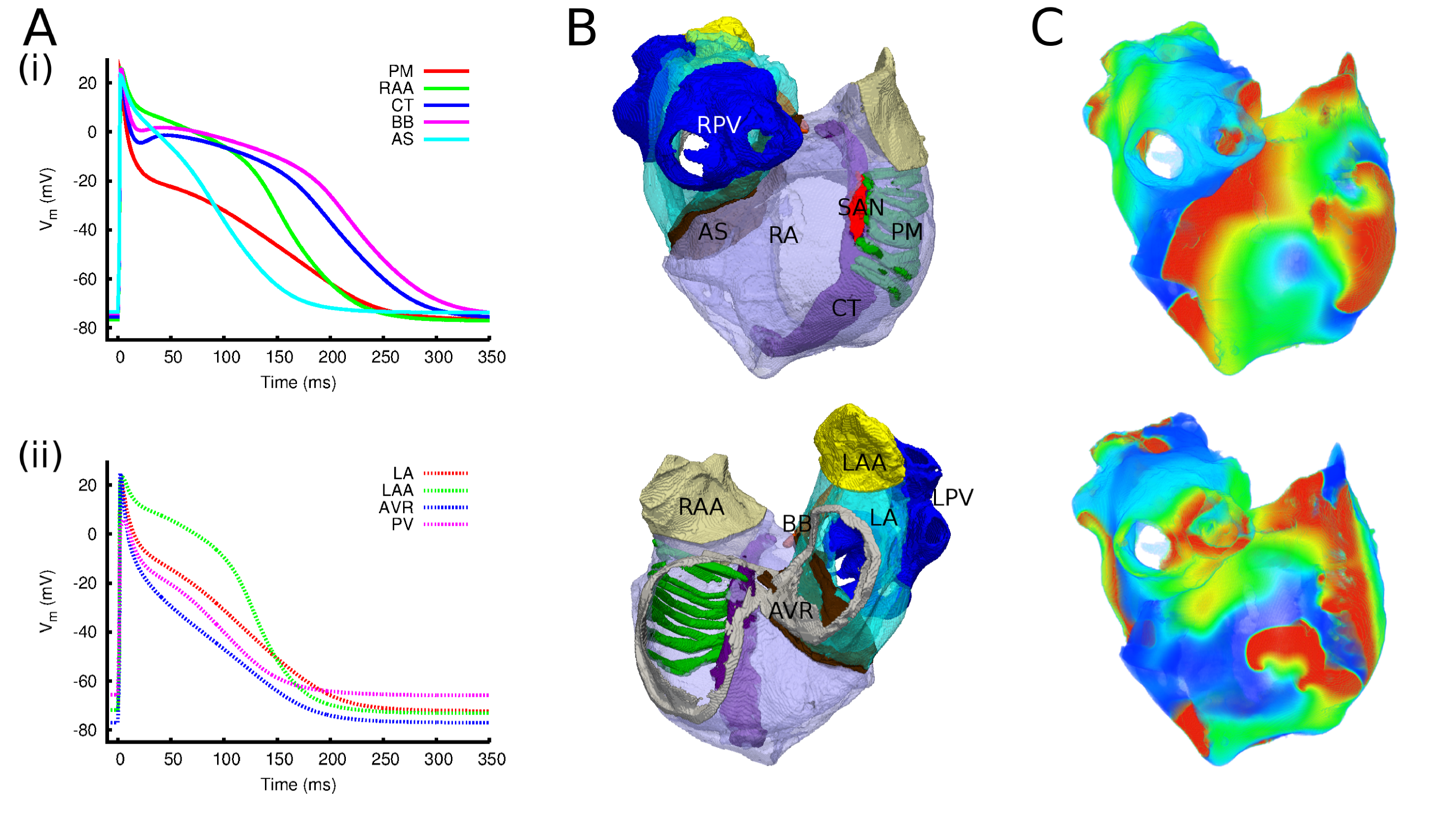
A new direction for the research of my group is studying the Cardiac Conduction System and its role in both normal and pathophysiological function.
Currently funded projects based in the lab
Remodelling of structure-function relationships underlying cardiac dysfunction in ageing
Source: MRC Career Development Award
Total value: £1,504,340
Tenure: October 2021 - October 2026
Description: This project brings together many of the different areas of research in our group. It will use a combination of simulations and experiments to characterise
the nature of ageing-associated remodelling of the structures of cardiac cells and tissues, and mechanistically link these remodelled structures to emergent dysfunction.
Learn More
Optogenetic control of organelle “chatter” and effects on calcium dynamics in human cardiomyocytes
Source: Human Frontier Science Program
Total value: $1,200,000
Tenure: 2025-2028
Description:
Recent nanoscale imaging (electron microscopy and super-resolution techniques) reveals the intricacies of subcellular compartmentalization. Most of these imaging approaches are terminal in nature, thus not ideal for proving causal structure-function relationships. Optogenetic tools offer high spatiotemporal precision and constitute a valuable interrogation technique to dissect such relationships in live cells and tissues. Excitation-contraction coupling, central to cardiomyocyte functionality, is a prime example of an evolved structure-function driven phenomenon. In cardiomyocytes, the periodically organized contractile units – the sarcomeres - are intimately surrounded by the T-tubules, the SR, and mitochondria to enable swift ECC operation. While human iPSC-CMs lack some of these mature structural features (special efforts are needed to make them form T-tubules), they have well developed sarcomere organization and relevant calcium handling, thus representing a suitable experimental model to study human cardiac ECC. As exemplified by synapses, cell-cell coupling, caveoli and T-tubule arrangements, nanospaces are nature's way to realize rapid chemical and electrical communication. Such communication, calcium release "chatter", between organelles with calcium release-uptake capabilities, is theoretically possible, based on their proximity. Yet, it is virtually unexplored and underappreciated in the current ECC theories. We will contribute to the fundamental understanding of how organelle interactions in myocytes may modulate calcium release and cardiac wave dynamics.
A computational model of fibrosis and the cardiac conduction system: the next generation of virtual heart models for research and teaching
Source: NC3Rs PhD Studentship
Total value: £124,159
Tenure: June 2024 - May 2028
Description: In collaboration with the Universities of Manchester and Auckland, we will combine pre-clinical and clinical images of the human atria
with our approach for modelling fibrosis and spontaneous calcium release. The resulting model, which will include realistic image-based structure of the cardiac
conduction system, should provide a valuable platform for future exploratory and mechanistic studies to address the mechanisms of arrhyhmia in patients.
Revealing the role of cardiac conduction system remodelling in ageing associated dysfunction of the heart
Source: Commonwealth PhD Scholarship
Tenure: October 2024 - September 2027
Description: Remodelling of the cardiac conduction system (CCS) occurs during the normal ageing process and has been causally linked to life-limiting and
life-threatening ageing-associated dysfunction of the heart. Despite this importance, the details of how the CCS remodels in ageing, and the mechanisms by which this remodelling
promotes dysfunction, have yet to be fully elucidated. This project aims to quantify this remodelling and reveal these underlying mechanisms.
The CCS will be segmented from high-resolution reconstructions of the heart, and its remodelling quantified through comparison of young and aged samples.
Electrical activity will be recorded through optical mapping. These highly detailed structural datasets will enable construction of well-validated, multi-scale computational models,
which will be used to tease apart the various interacting structure-function relationships that underlie disease mechanisms.
On The Use Of Animals In Research
Like many, I am an animal lover. I therefore have a personal and professional interest in the “3Rs of animal research”
– the policy of replacement, reduction and refinement. The work I do – developing and applying computational models –
is one of the approaches which can help to achieve this long-term ambition.
However, I would like to make clear that it would not be possible to develop these models without data from animal
experiments, and moreover that the models are not yet in a sophisticated enough state to allow all new research questions
to be answered without the requirement to collect more data. We must make difficult decisions, and I am of the opinion
that advancing medical knowledge and technology to improve human health, quality of life and longevity world-wide is
an important and major challenge. It is under this motivation, and with a heavy heart, that I cannot commit to never using animals in research.
However, following my personal, professional and moral motivations for minimising the negative impact on animals,
throughout my research career I will make the following commitments:
- Wherever possible, to perform and devise research projects which do not require any new animal experimentation. Through either:
- Purely computational research;
- Research which reuses previously collected animal data to provide new scientific insight, thereby maximising the impact of the data collected;
- Research which uses only human patient data, where it is sufficient.
- To perform new animal experiments only where necessary: where the available animal and human data are insufficient, where the research question is critically
important, and where the potential impacts of the research are substantial.
- Where new animal experiments are performed, to make every effort to ensure that the maximum impact is achieved through collaborating with researchers
with interests outside the heart who may make use of other tissues.
- To attempt to advance the translatability and clinical relevance of the computational models used in the field, through the development of both new
methods to expand their scope and highly rigorous and experimentally validated models of specific species and cells. These models reduce the need for
new animal experiments in the short-term, and in the long-term it is hoped they can contribute to the complete replacement of animal research.
Through effective implementation of these commitments, I also hope to promote the development and application of animal free approaches in the wider field.
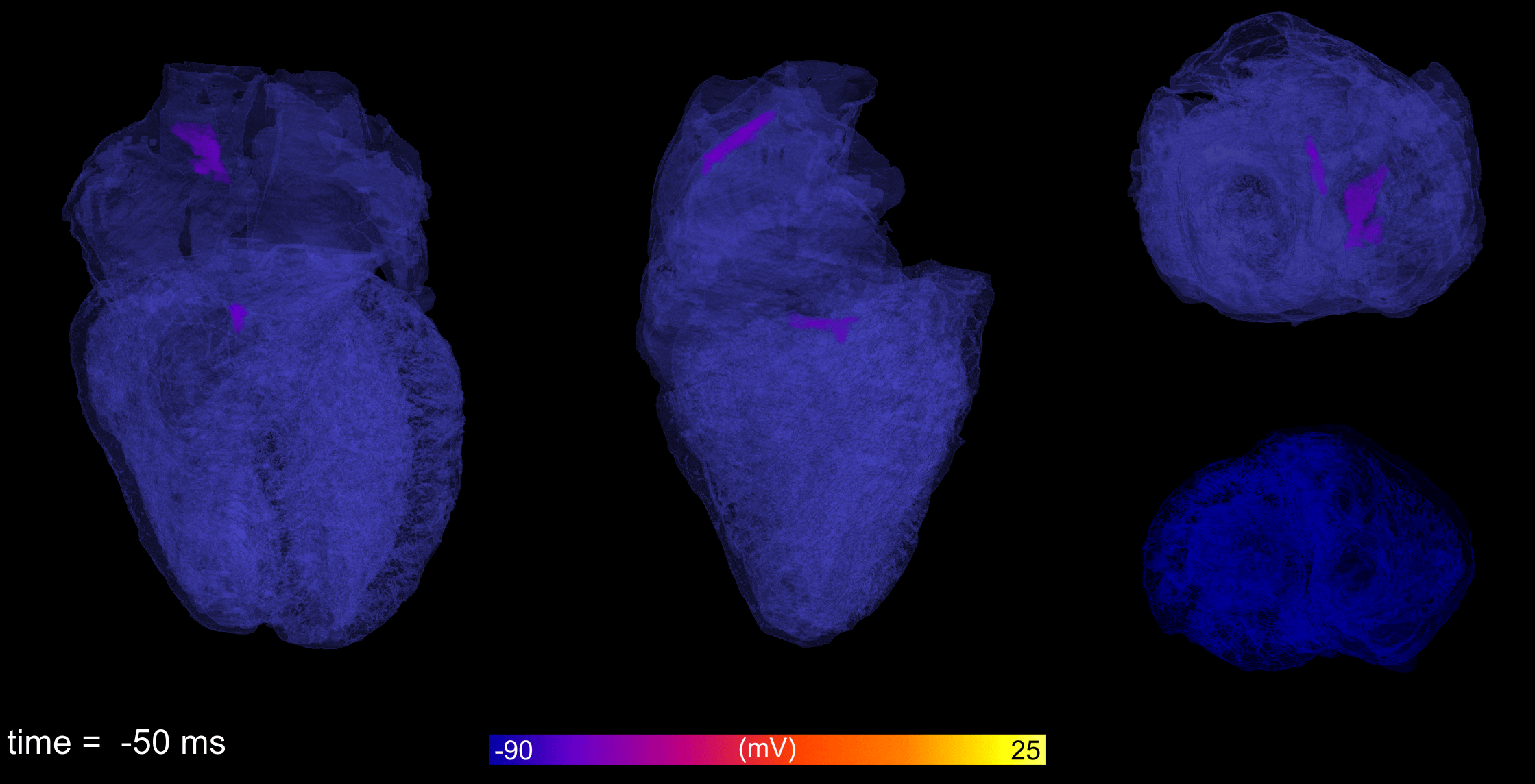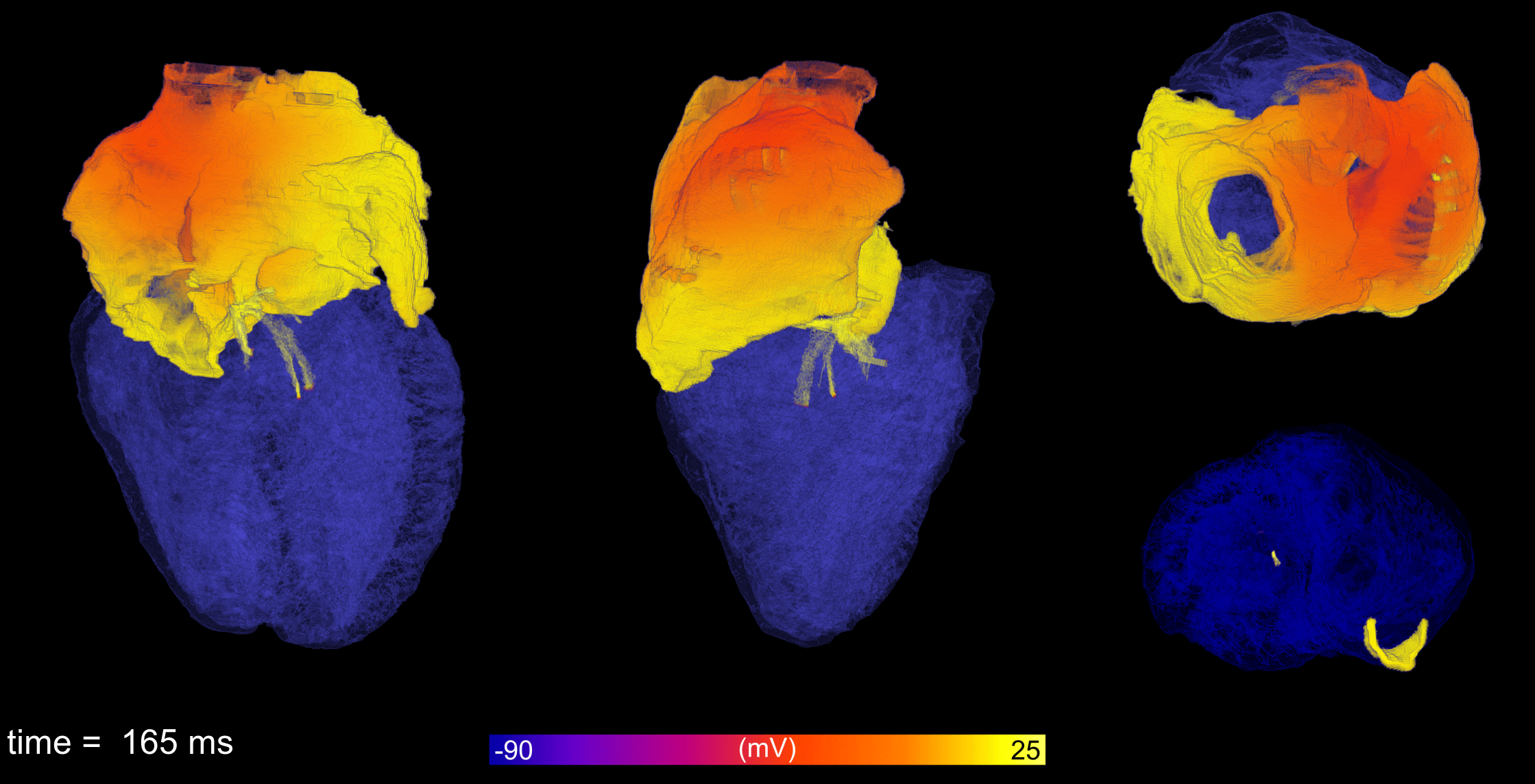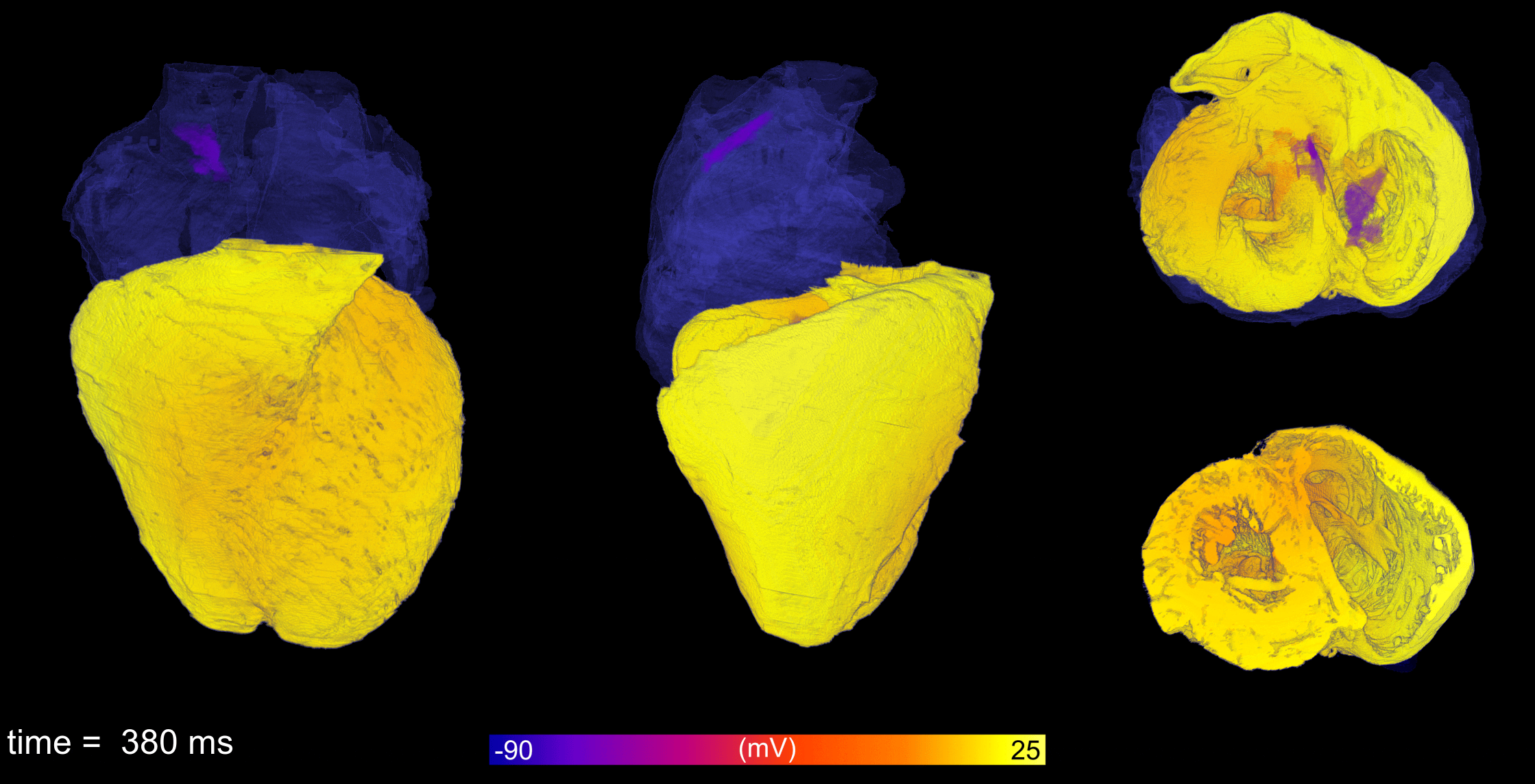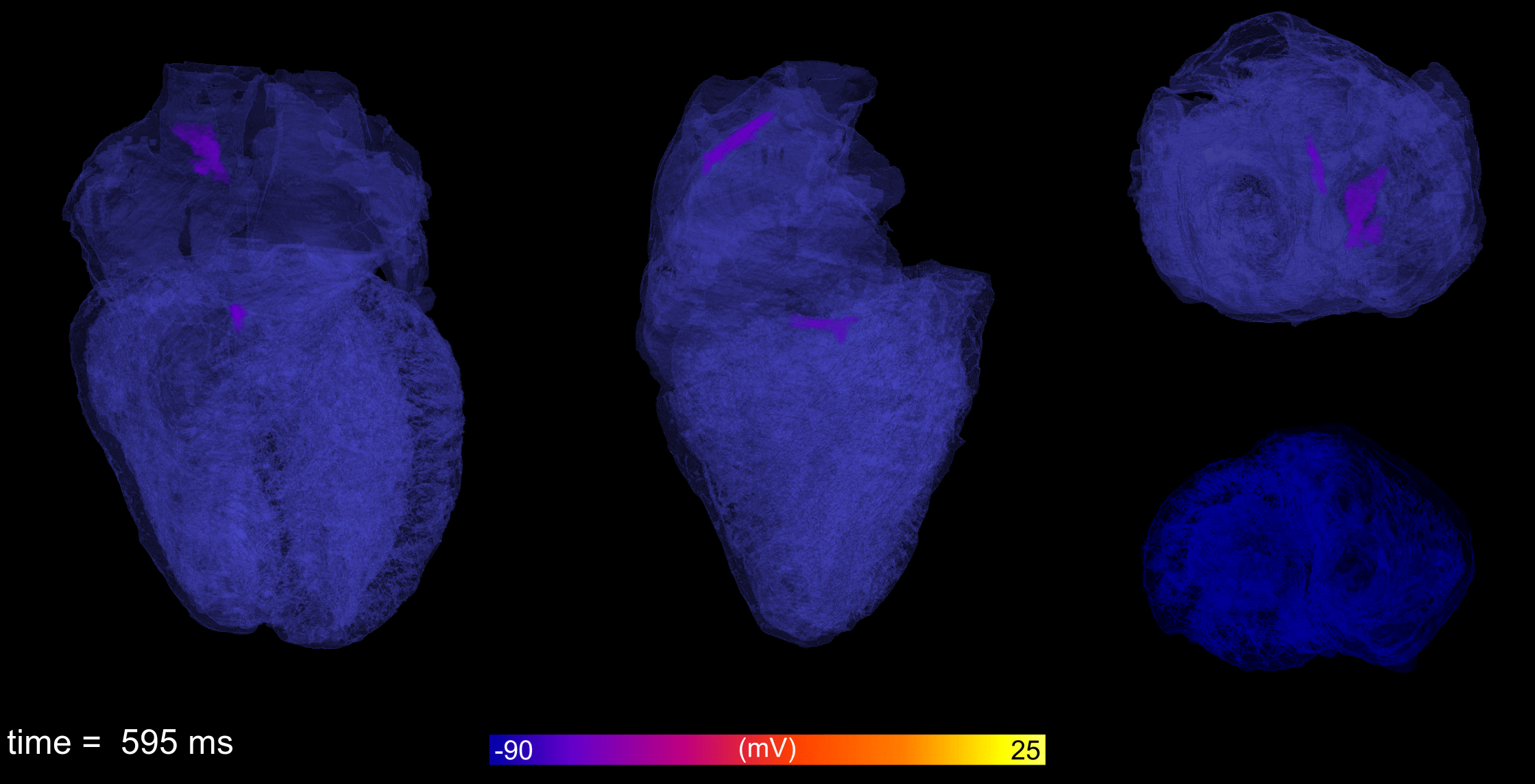
 Atrial fibrillation is the most common cardiac arrhythmia, leading to reduced cardiac output and increased
incidence of stroke and sudden cardiac death. Working with collaborators from the University of Glasgow and
King’s College London, computational models of atrial electrophysiology from cell-to-organ are developed and
applied to understand how the condition occurs, the details of its behaviour, and provide insight into how
it may be more effectively treated.
Atrial fibrillation is the most common cardiac arrhythmia, leading to reduced cardiac output and increased
incidence of stroke and sudden cardiac death. Working with collaborators from the University of Glasgow and
King’s College London, computational models of atrial electrophysiology from cell-to-organ are developed and
applied to understand how the condition occurs, the details of its behaviour, and provide insight into how
it may be more effectively treated.