Concept: The Cardiac Conduction System
This refers to the specialised system within the heart which ensures it contracts in a coordinated manner to give the most efficient pumping function. By initiating the heartbeat in a specialised pacemaker region which spontaneously generates Action Potentials, and then conducting the excitation along specialised networks which can either delay or accelerate the propagation, a uniform and controlled contraction can be maintained.
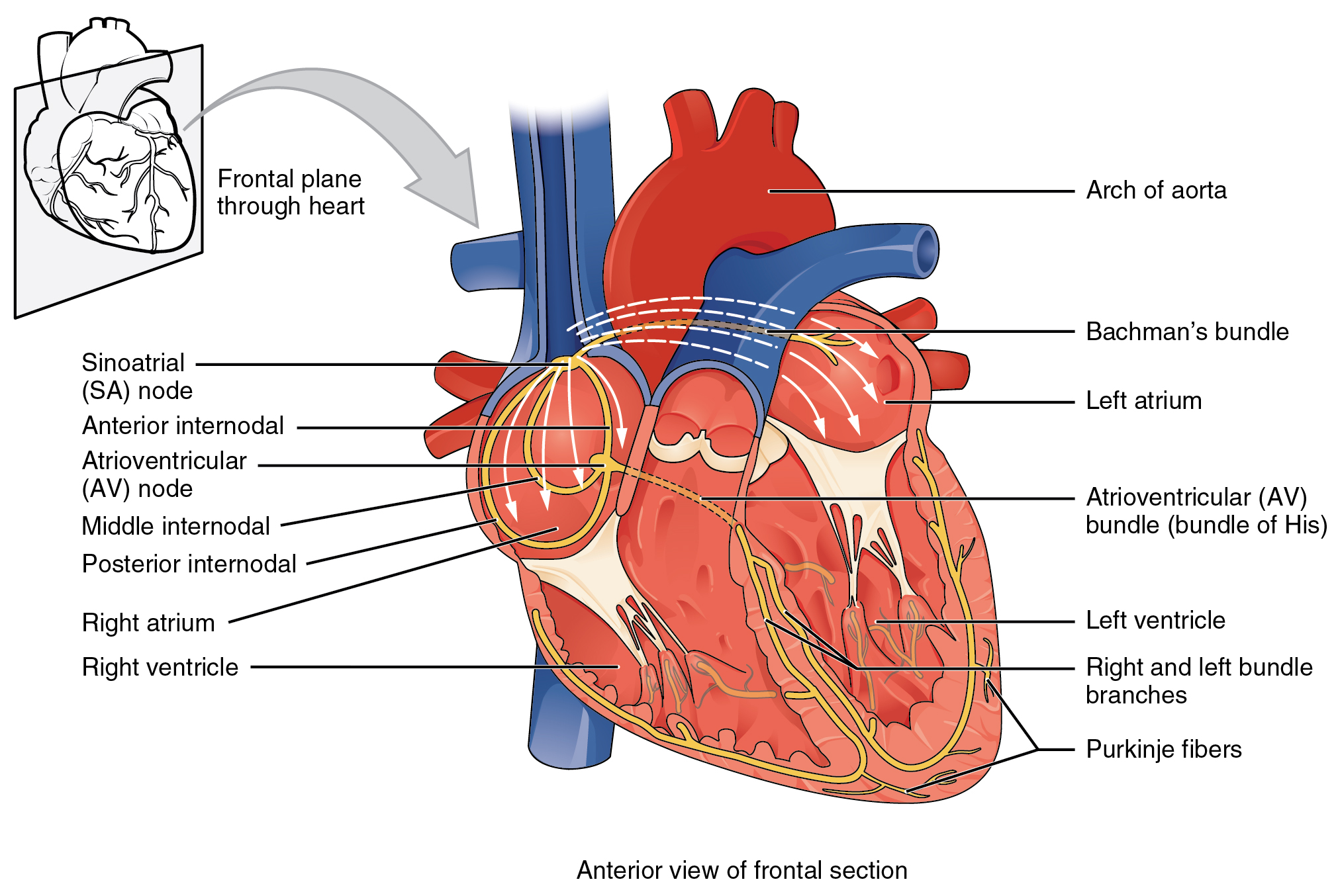 Illustration of the cardiac conduction system. Source: here (public domain)
Attribution: OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)]
Illustration of the cardiac conduction system. Source: here (public domain)
Attribution: OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)]
- The electrical excitation is initiated in the "sino-atrial node", the natural pacemaker, located near the top of the right atrium.
- The excitation is conducted along preferential pathways in the atria (the upper chambers of the heart) and down to the "atrio-ventricular node", the only electrical connection between the atria and ventricles .
- As excitation spreads throughout the atria, they contract and the ventricles fill with blood.
- Conduction is delayed in the atrio-ventricular node to allow time for the ventricles to fill.
- After this delay, excitation is conducted rapidly to the apex (the bottom) of the ventricles, through the bundle of His, before quickly spreading through the Purkinje Fibres throughout the ventricle. This supports near-simultaneous activation of the entire ventricular mass.
- The heart then undergoes repolarisation (returning to the non-excited state), allowing the atria and ventricles to fill in preparation for the next beat.
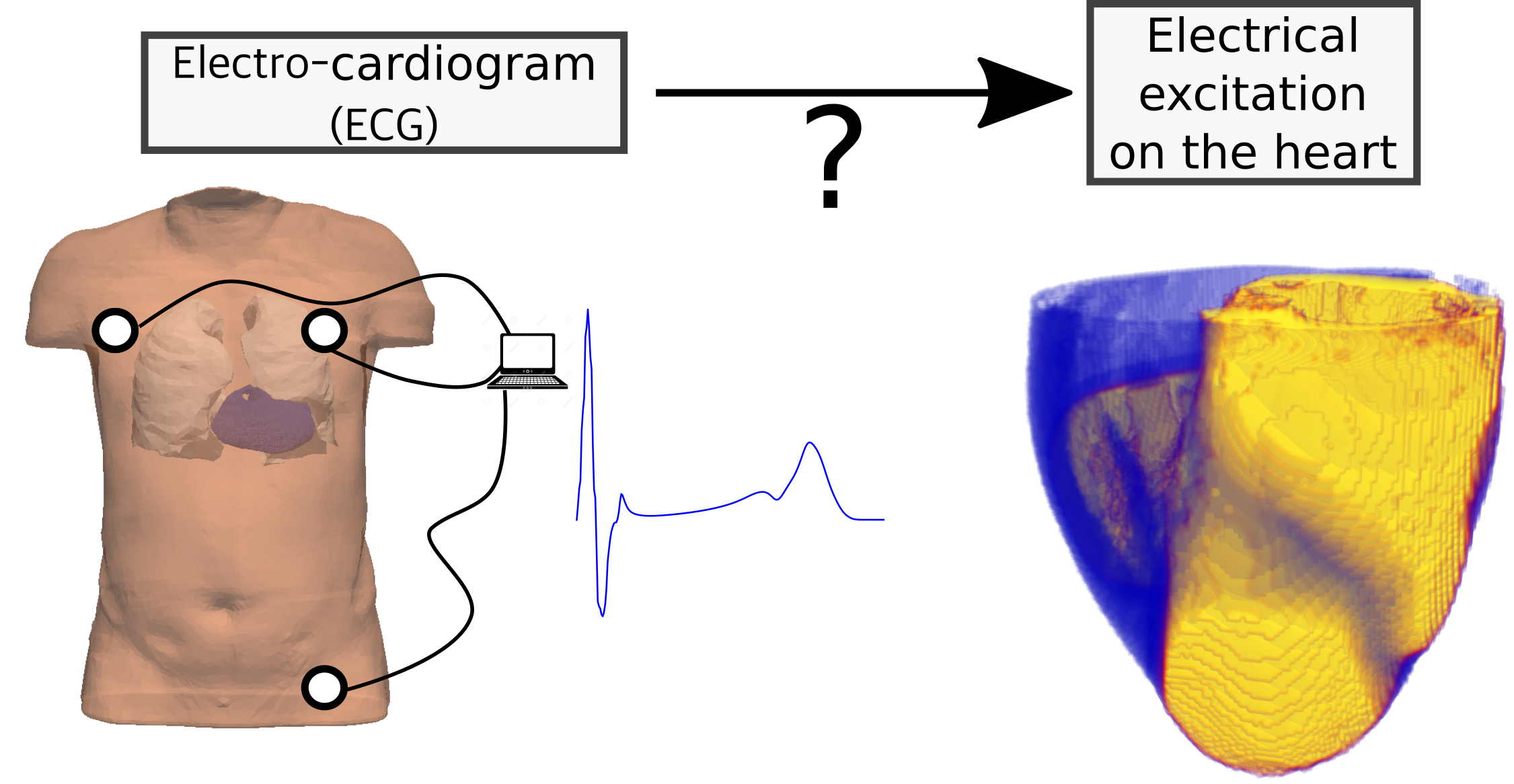
 Image adapted from
Image adapted from 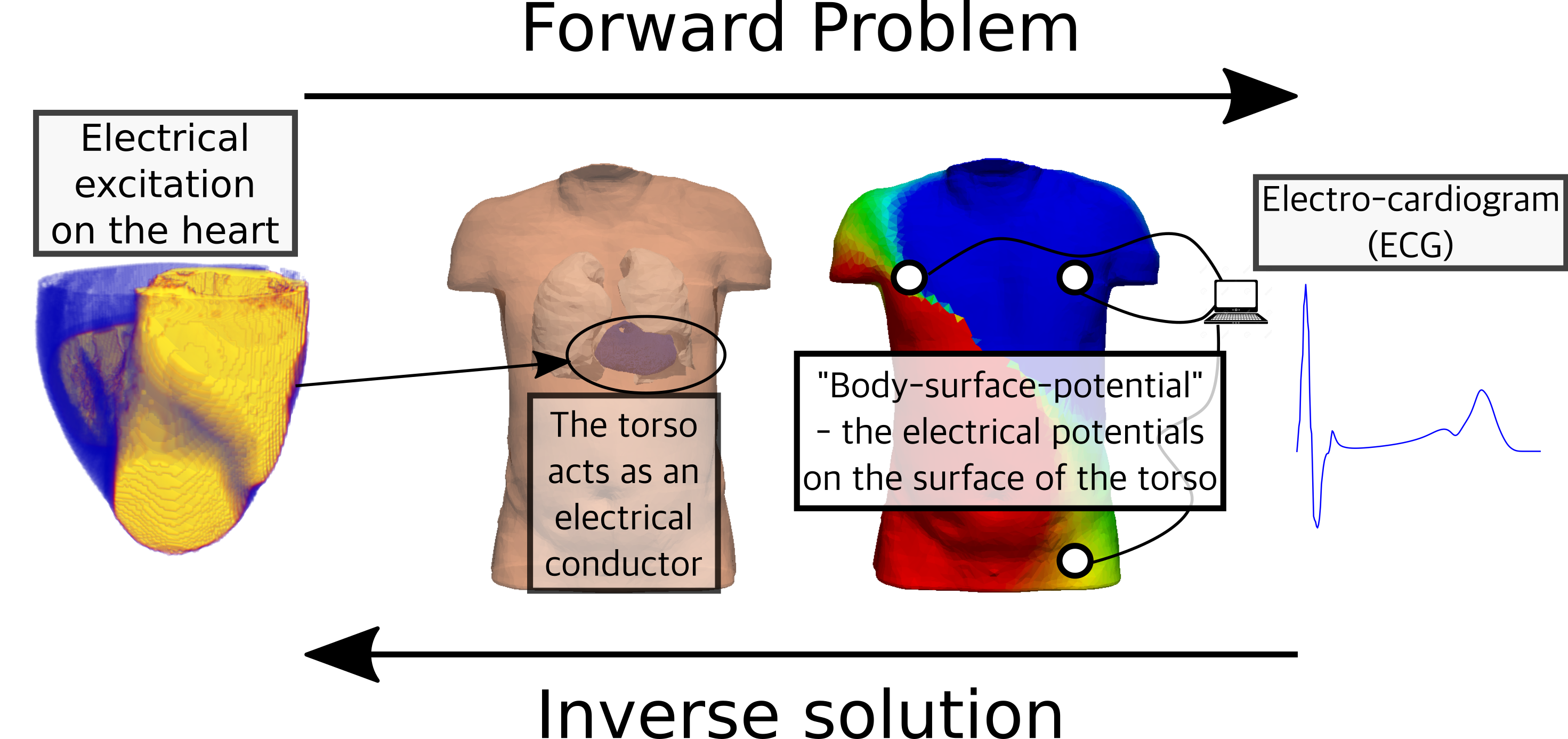 Illustration of the concepts of the Forward Problem and Inverse Solution. The Forward Problem
attemtps to predict the Body Surface Potential resulting from the electrical activity of the heart;
the Inverse Solution attempts to reconstruct the electrical activity of the heart from knowledge about
the Body Surface Potential.
Illustration of the concepts of the Forward Problem and Inverse Solution. The Forward Problem
attemtps to predict the Body Surface Potential resulting from the electrical activity of the heart;
the Inverse Solution attempts to reconstruct the electrical activity of the heart from knowledge about
the Body Surface Potential.
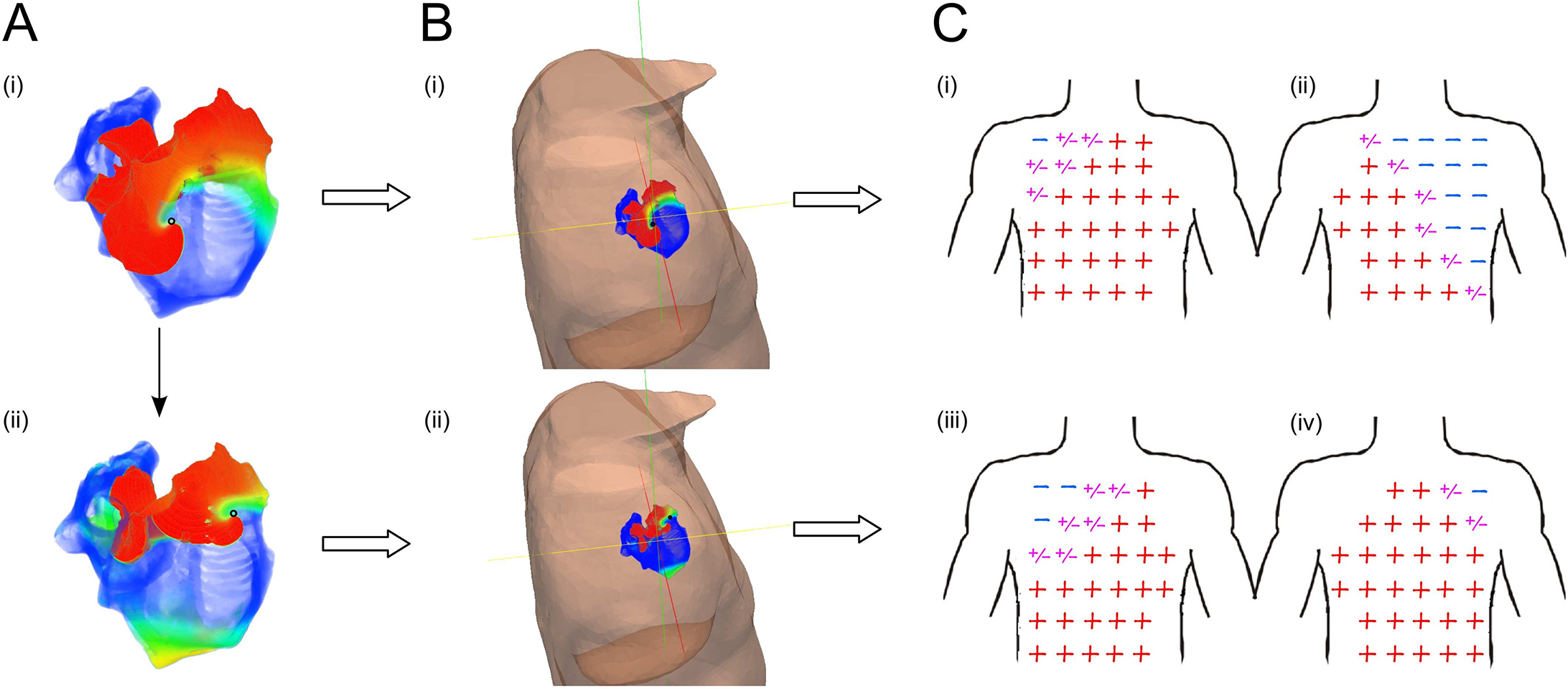 Figure: Illustration of the correlation between the source of excitation in the atria
and the polarity (orientation) of the P-wave at different locations on the body. Image
and data from
Figure: Illustration of the correlation between the source of excitation in the atria
and the polarity (orientation) of the P-wave at different locations on the body. Image
and data from
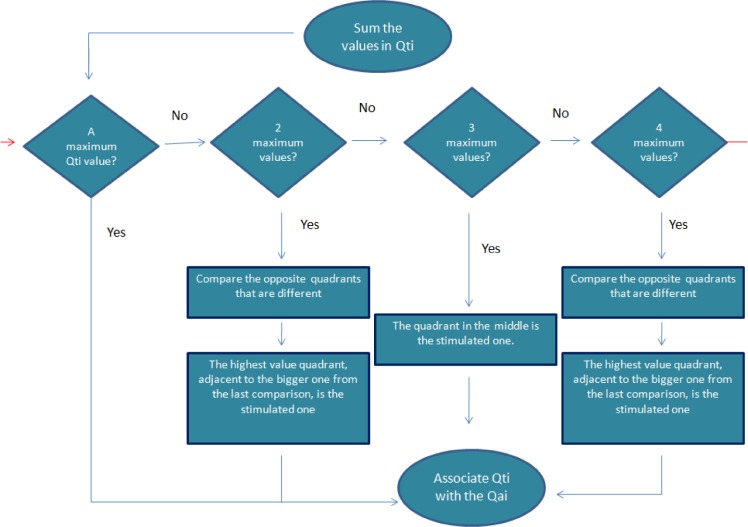 Figure: Algorithm to correlate ECG P-wave morphology with the source-location on the heart.
Image and data from
Figure: Algorithm to correlate ECG P-wave morphology with the source-location on the heart.
Image and data from
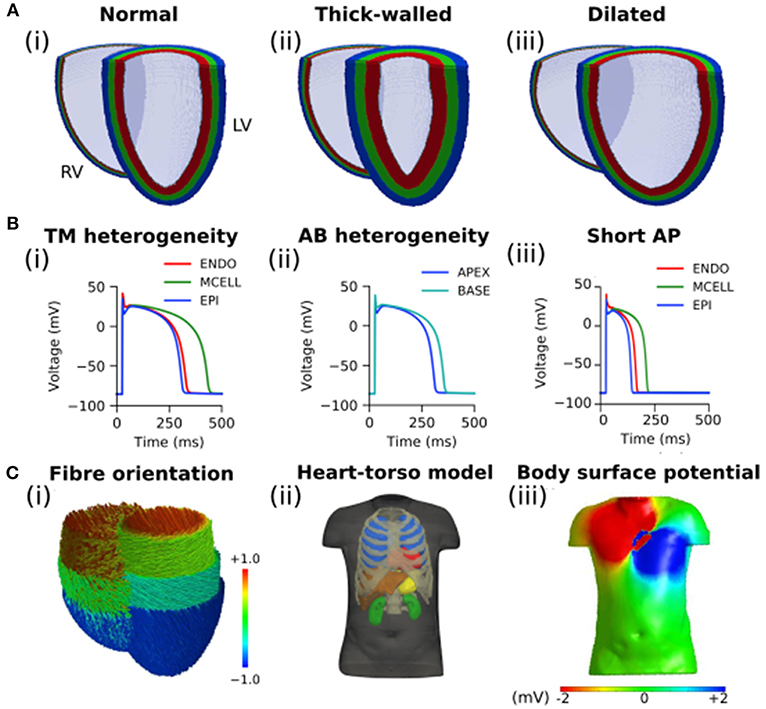 Figure: Models used to simulate the impact of heart size on the reconstructed potential maps.
Image and data from
Figure: Models used to simulate the impact of heart size on the reconstructed potential maps.
Image and data from
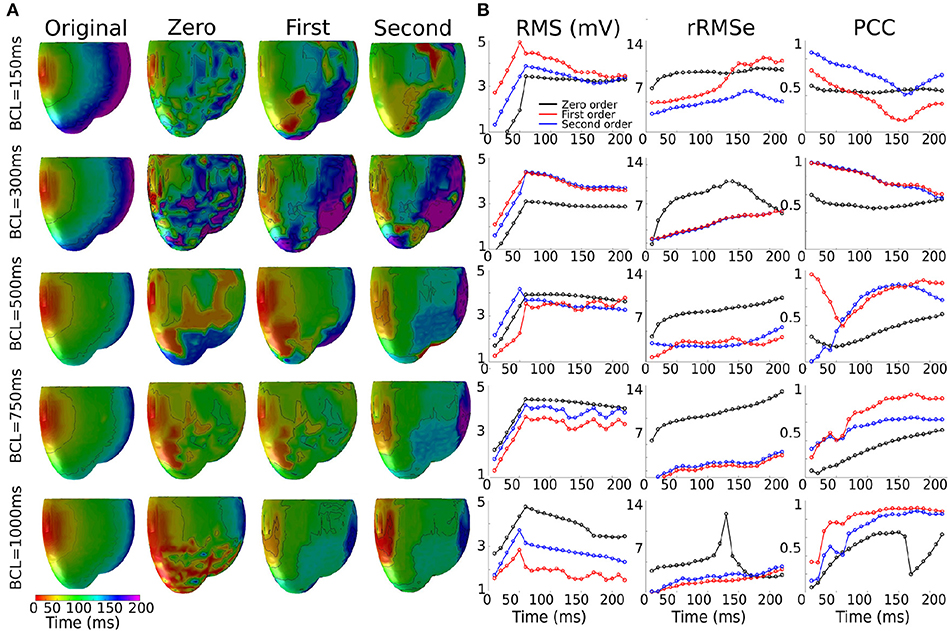 Figure: Extent of the errors and correlations over the course of ventricular activation at different
pacing rates.
Image and data from
Figure: Extent of the errors and correlations over the course of ventricular activation at different
pacing rates.
Image and data from